1. Introduction
The design of prostheses for the visual system presents a challenging problem for the biomedical engineer and neuroscientist. Great progress has been made in the miniaturization of prosthetic device electronics; however, development lags in the construction of practical stimulation interfaces with the visual system. The anatomy of the visual system is highly evolved and massively parallel in design. The optimization of neural interfaces to each level of the visual system presents different challenges and benefits. These problems cannot be solved by the methods of electronic circuit design or neuroscience alone, but by a hybrid of the two that adapts to the complex organization of natural neural systems. A variety of excellent reviews have appeared on visual prostheses (e.g. Margalit et al (2002, 2005), Hetling and Baig-Silva (2004), Tehovnik et al (2005), Weiland et al (2005)). This review departs from past neuroscience and engineering reviews of visual prostheses in that it deals primarily with actual problems encountered interfacing electronic devices to neurons of the visual system; encompassing anatomical, electrochemical, neuroethology, vascular, surgical, disease and physiological issues. References and documentation are supplied to the dimensions of human (or primate) visual system structures.
For prosthesis design, it is critically important to understand how natural vision operates in normal individuals and also in the severely visually impaired. This review covers what is known about the organization of the major target areas in the human brain for visual prostheses, the physiology and microanatomy of these areas from an engineering perspective, and issues involved in selectively stimulating neurons at each level of the visual system. Patients with a retinal disease called retinitis pigmentosa (RP) form a large portion of the prosthetic implant candidates currently undergoing clinical trials (Veraart et al 1998, Delbeke et al 2002, Humayun et al 2003,Chow et al 2004, Mahadevappa et al 2005). The review discusses how the visual areas are affected by RP and describes the visual behavior of end-stage RP patients in detail to assist in understanding their visual capabilities and needs.
2. Normal retinal function
In normal vision, light from the external visual environment enters the eye and is focused by the cornea and lens optics to form an image at the back of the eye on a sensory layer termed the ‘retina’ (figure 1). The retina is a thin layered network of neurons that contains light-sensitive cells called photoreceptors (figure 1 inset).
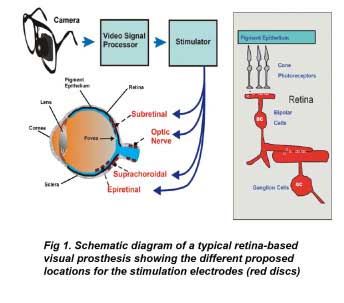
In the above figure, the retina and optic nerve are shown in blue. The electrodes of epiretinal prostheses lie closest to the retinal ganglion cells, while those of subretinal prostheses lie in the outer retina closest to the pigment epithelium. The electrodes of suprachoroidal or extraocular prostheses lie outside the retina behind the pigment epithelium. The stimulation electrodes of optic nerve prostheses are placed on the retinal optic nerve directly. Inset right shows the general structure of the retina showing the light-activated pathway from photoreceptors to ganglion cells.
Each point of light in visual space is focused by the eye’s optics on a corresponding point on the retinal surface, whose local intensity is encoded by retinal photoreceptors (e.g. figure 2(b)). Each neuron in the retinal network receives input from retinal photoreceptors within a spatial region that defines its ‘receptive field’.
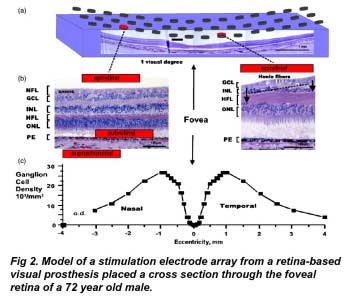
Fig2a shows a 15 × 3 array of epiretinal disc electrodes (250 µm diam.) is shown spanning the central retina along the nasal–temporal axis. Shrinkage is uncorrected. (Fig 2b) Cross sections of foveal retina with different stimulus electrode configurations (red). The thickness of the nerve fiber layer (NFL), Henle fiber layer (HFL) and ganglion cell layer (GCL) can vary significantly with retinal eccentricity. Left: in the nasal retina, a thick nerve fiber layer of ganglion cell axons covers the local ganglion cell stimulation targets from an epiretinal stimulus electrode. A subretinal electrode could better stimulate local inner retinal neurons but may occlude oxygen diffusion by choroidal capillaries adjacent to the pigment epithelium (PE). A suprachoroidal stimulus electrode is less invasive to the retina, but is located behind the resistive barrier of the pigment epithelium. Right: on the slope of the foveal pit, the nerve fiber layer is thin and the cone photoreceptors are displaced from their postsynaptic bipolar cell targets in the inner nuclear layer (INL) by their long fibers of Henle (arrows). (Fig 2c) Average density of ganglion cells across the nasal-temporal axis of the human fovea. Note the central fovea, a target of prosthesis stimulation, is devoid of ganglion cells. Ganglion cells receiving synaptic input from central foveal cone photoreceptors are displaced laterally from the fovea center, reaching a peak density at 1 mm retinal eccentricity. od: optic disc. (Adapted from Curcio and Allen 1990). ONL: outer nuclear layer (photoreceptors).
Two photoreceptor types in the outer retina transduce the light signal: rods, whose high sensitivity to light aids in vision in the dark, and cones, which can adapt to higher diurnal light levels. The cones also encode human color vision, and three spectral subtypes are present in humans. In normal retinal function, light strikes the outer segments of photoreceptors and is absorbed by visual pigment proteins named opsins, resulting in the isomerization of their chromophore 11-cisretinal. Through a complex biochemical process, opsin activation by light results in the closure of depolarizing ion channels normally open in the photoreceptor membrane, which causes the membrane potential to hyperpolarize and results in a reduction in synaptic neurotransmitter release. The neurotransmitter released at the photoreceptor synaptic terminal is the excitatory amino acid glutamate.
Rod and cone photoreceptors send the light signal through synapses in the outer retina onto two different classes of bipolar cells located in the inner retina. These two cell classes have synaptic receptors for glutamate that produce opposite responses to light. OFF-center bipolar cells hyperpolarize to light like their photoreceptor inputs, while ON-center bipolar cells depolarize to light. In primates, there are ten different bipolar cell types (Boycott and W¨assle 1991), with the midget bipolar cells being the most common type involved in central vision. In lower vertebrates, different bipolar cell types respond to different ranges of contrast in the visual scene (Burkhardt and Fahey 1998) and this may partly account for the many primate bipolar cell types. The stimulated bipolar cells synaptically release glutamate, which depolarizes both ON-and OFF-center amacrine and ganglion cells in the inner retina.
On average, one million ganglion cells are found in the inner retina of humans, but this number varies up to two-fold between individuals (Curcio and Allen 1990). In primates and humans, the retinal ganglion cells are thought to comprise between 13 and 18 different types (Polyak 1941,Kolb et al 1992, Dacey and Packer 2003, Dacey et al 2005). Upon excitation by bipolar cells, retinal ganglion cells generate action potentials. These action potentials propagate down the ganglion cell axon, exit the eye at the optic disc, and excite the visual areas of the brain through the optic nerve. The presence of functional ganglion cells is critical to the operation of all retina-based visual prostheses. In humans, about 90% of the ganglion cells in the central visual area are a single class of ganglion cell termed ‘midget’ or P-type and are thought to encode color and high spatial resolution vision. These cells form the ‘parvocellular’ pathway to the visual areas of the brain. A second sparser class of ganglion cells, termed ‘parasol’ or M-type, is sensitive to movement and low-contrast stimuli. These cells form the ‘magnocellular’ pathway. The innermost retinal layer is the ‘nerve fiber layer’ formed from the crossing fascicles of unmyelinated axons from more distal retinal ganglion cells converging on the optic disc (figure 2(b)).
3. Role of the fovea in visually guided behavior
In human and primate retinas, a small central area called the ‘fovea’ is specialized for high resolution vision. The fovea plays a key role in visually guided behavior. Through fixational eye movements, we constantly direct the fovea to analyze the fine details in a visual scene. A human fovea is shown in cross section in figure 2(a). The foveal pit subtends the central 3◦ of vision, and occupies a retinal surface area roughly 1 mm in diameter (1◦ of visual angle is equal to 275– 300 µm at the retina (e.g. Drasdo and Fowler (1974) figure 4). Retinal thickness of the parafovea averages around 150 µm (Neubauer et al 2001); however, in the center of the foveal pit the retina becomes extremely thin as only cone photoreceptors are present. The density of cones is highest at the fovea: peaking at a density of 199 200 ± 87 200 mm−2 (Curcio et al 1990). In the fovea all second-and third-order retinal neurons are displaced centrifugally in an annular ring away from their foveal cones. This structural pattern is formed early in post-natal life (Hendrickson and Yuodelis 1984) making replacement by stem cells difficult. Cone photoreceptor cells synaptically contact bipolar and horizontal cells through elongated processes termed the ‘fibers of Henle’ which form laterally-displaced synaptic endings (figure 2(b)). It is the fovea and its surrounding region that most retinal prosthetic devices strive to stimulate. Stimulation of the peripheral retina may require patients to learn eccentric eye fixation techniques.
Like their photoreceptor inputs, roughly 50% of all ganglion cells in humans are localized within 4.5 mm of the fovea (Curcio and Allen 1990). However, since the fovea contains only cone photoreceptors, ganglion cells receiving foveal cone connections are also displaced centrifugally toward the periphery, an average of 0.37 mm away from the center (figure 2(b)) (Sjostrand et al 1999). Single midget bipolar cells receive input from single foveal cones, which contact midget ganglion cells. At ∼1 mm from the fovea, the ganglion cell density peaks at 25–30 000 cells mm−2.This density then slowly declines so that at 3 mm from the fovea, the ganglion cell density is less than 1/5 of the peak. How these displaced foveal ganglion cells are locally stimulated is critical to the generation of effective pattern vision by retinal prostheses.
4. The prosthetic candidate (visually-impaired patient)
An important issue in visual prosthesis design is understanding the nature and visual needs of the implant candidate population. The majority of these visually impaired patients suffer from diseases within the eye itself; particularly degenerative retinal diseases that effect central visual function. A large patient group currently involved in clinical trials of retinal prostheses have the end-stages of a progressive photoreceptor degeneration known as ‘retinitis pigmentosa’ or RP. A second potential patient pool have end-stages of the retinal disease ‘macular degeneration’ (Margalit and Sadda 2003). This is a disease in which abnormal blood vessels grow under the central retina, leak fluid and blood and eventually cause degeneration, and scarring. Finally, a small percentage of the severely impaired patients have strokes of the optic chiasm, optic nerve atrophy, or optic nerve neuritis. For these patients, the retina is non-functional and the only remaining option currently under study is a prosthesis that stimulates the visual cortex. To date at least 23 different groups are designing visual prostheses (Hessburg and Rizzo 2007, Wickelgren 2006).
RP is a genetically-based retinal disease that initially presents as a degeneration of rod photoreceptors which results in a loss of night vision. Pigmented lesions with the appearance of ‘spicules’ are seen in the retina upon ophthalmoscopic examination. RP patients often report shimmering or flashes of light in their visual fields. Peripheral vision is gradually lost resulting in a narrow central visual field some 3–10◦ in diameter. In its end-stage, RP can result in a near complete degeneration of the retinal photoreceptors. A few isolated islands of light sensitivity may remain in the peripheral retina. Disease progression is highly variable, and only RP patients who are severely visually impaired would potentially benefit from the current visual prostheses (e.g. Berson et al (2002), Grover et al (1998, 1999), Fishman (1978), Milam et al (1998)). These severely afflicted patients are at best only able to detect the presence of light or hand motion in a few isolated areas of the visual field (Grover et al 1998). The retinas of RP patients exhibit a foveal slope (Stone et al 1992), however their foveolae are abnormal. In cross section, they typically exhibit two or fewer rows of photoreceptor cell bodies unlike the five–eight rows normally present (table 2 in Stone et al (1992)).
End-stage RP, while ostensibly causing degeneration of foveal photoreceptors can also induce secondary degeneration of the foveal ganglion cells, a critical stimulation target of retinal prostheses (Stone et al 1992, Santos et al 1997, Humayun et al 1999a). Histological studies of the macula indicate that while 78% of the inner nuclear layer neurons survive, only 30% of the ganglion cells remain and in a few cases there can be a total loss (Santos et al 1997, Humayun et al 1999a). Although inner nuclear layer neurons and ganglion cells are reduced in RP patients, the retinal neurons remaining can elicit the sensation of light in patients when electrically stimulated (Humayun et al 1996, Rizzo et al 2003a, 2003b, Feucht et al 2005). In human retinitis pigmentosa, the normally layered retina tends to degenerate with the few surviving rods sprouting neurites that extend to the inner limiting membrane (Li et al 1995), and abnormal neural connections are formed by amacrine and horizontal cells (Fariss et al 2000). Thus a retinal prosthesis in an end-stage RP patient’s eye may potentially stimulate a retina with unusual connections not seen in normal individuals. Animal models of RP, such as the RCS rat and the Rd mouse (Potts and Inoue 1969, Marc and Jones 2003) exhibit similar patterns of retinal neuron reorganization (Strettoi and Pignatelli 2000, Jones et al 2003,Marc et al 2003) and are actively being studied by prosthesis researchers (e.g. Suzuki et al (2004)).
Currently, mutations in over 30 different retinal genes have been implicated in RP making a simple genetic cure for the disease difficult to achieve (Wang et al 2005, Rivolta et al 2002, Milam et al 1998). The genetics of RP are complex, as the disease can be autosomal-dominant, autosomal-recessive, X-linked, or of non-familial etiology (simplex). About 60% of RP cases still have no known genetic cause (Wang et al 2005, Milam et al 1998). The degree of visual loss in RP is mildest in cases of autosomal-dominant disease and most severe in cases of X-linked disease (Fishman, 1978). Posterior subcapsular cataracts of the lens, commonly associated with late-stage RP, can confound assessment of patient visual performance. While it is likely that genetic vectors or stem cell technology may ultimately repair some forms of RP (Acland et al 2001), the sheer number of mutations involved precludes simple therapies and may require intact rod precursors (MacLaren et al 2006). However, visual cortex function is often unaffected in RP patients (e.g. Jacobson et al (1985)). Thus for these advanced patients, a visual prosthesis may be the only option.
5. Retinal visual prostheses
Retinal prostheses attempt to integrate into the diseased retinal network of the visually impaired patient’s eye in order to create the percept of pattern vision. The typical prosthesis uses a camera, mounted either on glasses or in the eye to provide the visual input (figure 1). The camera output is sent through a video processor and neural stimulator to stimulus electrodes embedded in the patient’s eye to create the sensation of vision. Unlike visual prostheses in the brain, retinal prostheses are readily accessible surgically and can be monitored through the eye. Currently, most prostheses types proposed use electrical current to depolarize neurons.
5.1. Retinal prostheses relying on electrical current stimulation
Electrical stimulation of the globe of the human eye has long been known to elicit an artificial sensation of light termed an electrophosphene (e.g. Purkinje (1823)). Further research showed the electrophosphenes were of ocular origin (e.g. Finklestein (1894)), and finally due to activation of the retina itself (Granit and Helme 1939, Brindley 1955). The first retinal prosthesis for the blind was proposed by Tassiker (1956). It consisted of a light-sensitive selenium photodiode cell placed behind the retina. There are three possible mechanisms by which electrical stimulation can activate retinal pathways to elicit electrophosphenes in blind patients. First, electrodes can depolarize and form action potentials in ganglion cell axons. Second, electrical currents can depolarize and form action potentials in local ganglion cells directly. Finally electrical currents can depolarize cells in the retinal network such as bipolar or amacrine cells which propagate the visual signal to ganglion cells. The electrochemical properties of stimulating electrodes containing Pt or Ir metal have been extensively studied (e.g. Brummer and Turner (1977), Rose and Robblee (1990), Robblee et al (1983)). As with most neural stimulation, stimulation protocols are limited to charge balanced biphasic current pulses of short duration (0.05–5 ms) to prevent metal electrode dissolution. Four different locations are currently being evaluated for the stimulus electrodes in retinal prostheses: epiretinal, subretinal, suprachoroidal and the optic nerve (figure 1).
5.2. Epiretinal stimulation electrodes
In the epiretinal configuration, the stimulation electrode array is placed in the vitreous cavity directly over the inner retinal surface in close proximity to the ganglion cell bodies. Advantages of this design include that it is less invasive to the retina, does not occlude the retinal vasculature, and can be monitored ophthalmoscopically. In acute clinical trials with an epiretinal electrode, several groups have shown that biphasic current pulse stimulation elicited an electrophosphene in blind volunteers (Humayun et al 1996, Rizzo et al 2003a, Feucht et al 2005). Higher frequency stimulation of 40–50 Hz gave non-flickering maintained percepts (Humayun et al 1999b,see also Brindley (1955)). Currently, clinical trials of a 4 × 4array of silicon-insulated Pt disc epiretinal electrodes (250–500 µm diam.) are ongoing in RP patients (Mahadevappa et al 2005). The electrode array is held against the retinal surface with an implanted titanium tack. A camera and video processor connected to a modified cochlear implant pulse generator is used to provide visual stimulation (Humayun et al 2003). In the patients, biphasic stimulation of most electrodes elicited electrophosphenes (threshold 24–702 µA, 1 ms, cathodic first), and some were able to detect movement (Humayun et al 2003, Mahadevappa et al 2005). The electrophosphene ‘visual field’ map of one implanted individual has been reported (Humayun et al 2003); however it is unclear how well the map corresponds to the actual retinal electrode locations (see also Rizzo et al (2003b)). Currently, several epiretinal prostheses are under development incorporating 49–60 electrodes to improve spatial resolution (Hornig and Richard 2006, Javaheri et al 2006, Wickelgren 2006).
5.3. Subretinal stimulation electrodes
Stimulating electrode arrays are also being tested in the subretinal space of visually impaired patients. This space is located between the pigment epithelium and the photoreceptor layer of the retina. The advantage of the subretinal stimulation location is that it can depolarize the remaining bipolar cells in the retinal circuits of RP patients, which may produce a more natural excitation of the ganglion cells. The disadvantages of these arrays may stem from the barrier they form between the choriocapillaris vasculature and the retina, leaving the retinal artery vasculature as the sole retinal oxygen source (see section 10). To date, two main types of subretinal devices are being tested: passively and actively powered stimulation arrays.
Passively powered subretinal stimulation arrays are currently being tested in clinical trials in RP patients (Chow et al 2004). Chow and Chow (1997) fabricated an implantable circular microchip 2 mm in diameter and 25 µm thick containing an array of 5000 silicon microphotodiodes with IrOx electrodes termed an ‘artificial silicon retina’ or ASR. Light passively strikes photodiodes in the array, which generate local electrical currents that activate the inner retinal synaptic circuitry to elicit the sensation of vision. Preliminary experiments with implanted ASRs in rabbit eyes using IR LEDs as stimuli suggested it was possible to record photodiode-evoked cortical potentials thought to originate from the stimulated retina (e.g. Peyman et al (1998)). However when stimulating humans with IR light in the dark, it is important to control for the IR spectral sensitivity of rods and long wavelength cones (see Griffin et al (1947), Stockman and Sharpe (2000), Pardue et al (2001)). A second group headed by Eberhardt Zrenner fabricated a similar series of passively powered silicone photodiode chips that were tested in pigs, rats, and chicken retinas (Zrenner 2002b). In animal studies, both groups found that the implant was well tolerated by the adjoining retina. However, Zrenner’s group has concluded that it is unlikely for single microphotodiodes to be able to generate sufficient current to activate local neurons in the retinal network using ambient light levels (Zrenner 2002a, Gekeler and Zrenner 2005). Their active microphotodiode implant incorporates adaptation circuits and external power sources (Gekeler and Zrenner 2005).
There are also problems in evaluating the effectiveness of the passive photodiode retinal implants. Unlike more complex prostheses, a passive photodiode array is chronically active and cannot be turned off, complicating metrics of clinical success due to lack of a non-functional control. Healing factors induced by the surgery could confound device effectiveness, as studies have shown that surgical wounds increase intrinsic retinal neurotrophins (Cao et al 1997, 2001, Sakai et al 1999). Healing factors appear to have a neuroprotective effect, slowing the natural photoreceptor degeneration in animal models of RP. Pardue et al (2005a, 2005b) have recently reported in an RP rat model that implantation of active or inactive ASR chips preserve photoreceptor nuclei equally.
The second type of subretinal stimulation electrode array under investigation is actively powered. This array design is currently in the prostheses under study by two groups; one headed in the US by Joseph Rizzo, and a second in Germany by Eberhard Zrenner. Both groups propose to provide power to their prostheses through telemetry coils typically mounted on eyeglasses (Gekeler and Zrenner 2005, Rizzo 2006). In the rabbit retina, stimulation with active subretinal electrode arrays is able to generate electrically evoked potentials in visual cortex with charge densities of as little as 10 µCcm−2 (Gekeler et al 2004), however higher stimulation thresholds have been found in pigs (100 µCcm−2) (Sachs et al 2005, Schanze et al 2006). Subretinal electrode stimulation with cathodal pulses may also be able to selectively stimulate ON-versus OFF-center ganglion cells in the rabbit retina (Jensen and Rizzo 2006), a critical feature for evoking electrophosphenes more biologically relevant to normal retinal function. Preliminary tests in RP patients with an active 4 × 4 subretinal array show they are able to perceive discrete visual phosphenes, lines, and a square (Wilke et al 2006, Wickelgren 2006).
5.4. Extraocular electrodes
Recently, several groups in Japan and Australia have performed experiments in animals using a stimulating electrode array located inside or on the surface of the sclera of the eye (Kanda et al 2004, Sakaguchi et al 2004, Nakauchi et al 2005, Chowdhury et al 2005). These designs have been termed ‘suprachoroidal’ ‘intrascleral’ or ‘extraocular’ electrode arrays. The advantages of these designs are that they appear to cause little risk of retinal detachment, do not occlude the choroidal vasculature, and are adjacent to the outer retina where the visual signal originates. Their disadvantages are that they are located behind the resistive barrier of the pigment epithelium (or R-membrane) which impedes electric current flow into the retina (e.g. Tomita et al (1960)). While the resistivity of the retina is quite low to electric current flow ∼40–60 Q cm2, the resistance of the pigment epithelium is 5–15-fold higher (e.g. frog: Tomita et al (1960), rabbit: Karwoski and Xu (1999), macaque: Heynen and van Norren (1985), Tsuboi et al (1987), human: Quinn and Miller (1992)).
Experiments in rabbits with suprachoroidal arrays suggest they are well tolerated in the sclera of the eye and no adverse ocular pathology was seen (Nakauchi et al 2005). Currently, it is unclear if the currents elicited by these electrodes will be adequate to evoke electrophosphenes in blind patients, however in RCS rats suprachoroidal electrode stimulation elicited evoked-potentials in the superior colliculus (Kanda et al 2004). In rabbit retinas, suprachoroidal electrode array stimulation elicited electrically evoked cortical potentials (an index of retinal function), but thresholds were 16× higher than with subretinal stimulation (Yamauchi et al 2005). These higher stimulus thresholds may be reduced with a scleralmounted penetrating retinal implant array currently under development (Gerding 2007).
5.5. Optic nerve electrodes
Stimulation electrodes have also been tested on the optic nerve (figure 1). A Belgium-based group has developed a series of four platinum discs (0.2 mm2) mounted in a spiral cuff electrode to stimulate sectors of the optic nerve. To date, it has been implanted in two RP patients (Veraart et al 1998, Delbeke et al 2002). Cuff electrodes, mounted on either the orbital or cranial sections of the optic nerve, were attached to a wireless subdermal stimulator located on the side of the head. In the single patient tested, optic nerve stimulation generated a field of phosphene ‘dots’ whose threshold, area and visual field location depended on pulse train duration and stimulus strength (single pulse threshold, 250 µA). Weaker/shorter stimuli generated more peripherally field percepts while stronger/longer stimuli resulting in more central fields (Delbeke et al 2003). While there is little trauma to the eye itself with such a system, it is unclear if optic nerve electrodes can elicit the discrete spatially adjacent phosphene fields needed for generating pattern vision given the large number of axons in the optic nerve.
6. Issues with retinal prostheses
6.1. Retinotopic stimulation in the fovea
Theoretically, it would be desirable to stimulate the visual system near the fovea in order to better exploit the remaining high resolution visual pathways. However, there are complexities with stimulating foveal ganglion cell receptive fields. To illustrate these issues, a human fovea is shown overlaid with a model array of epiretinal stimulating electrodes in figure 2(a). Ideally, stimulation by a single epiretinal electrode induces action potentials in the underlying local patch of ganglion cells which would be perceived by the patient as an electrophosphene originating from the overlying photoreceptors. However a problem arises: the central fovea contains no ganglion cells; only cone photoreceptors. Foveal ganglion cell bodies, a typical target of electrical stimulation by visual prostheses, are physically displaced around the edge of the foveal pit in a ring 0.4–2 mm in diameter at a peak density of 25 000–35 000 cells mm−2 (Curcio and Allen 1990). The density of ganglion cells across the fovea is shown in figure 2(c). In a single cross section through the foveal edge, ganglion cell bodies are stacked up to six–eight cells deep in a layer some 50–60 µm in thickness (Curcio and Allen 1990). This creates a problem for epiretinal visual prosthesis designs. The annular displacement of the central foveal ganglion cell bodies to the edge of the foveal slope distorts the assumption that electrode stimulation of local ganglion cell bodies generates a local electrophosphene percept. Conceivably, these distortion problems could be reduced by placing a subretinal prosthesis under the fovea, however this could occlude the sole blood supply to the central fovea (see section 10.1). Given the above issues and the variable degeneration found in RP patients, a complex remapping of prosthetic stimulus electrodes to foveal ganglion cell receptive fields may be required in order to generate a uniform ‘visual field map’ of electrophosphenes.
6.2. Selective stimulation of local retinal ganglion cells
An epiretinal prosthesis places stimulation electrodes near the inner retinal surface to induce action potentials in the underlying local patch of ganglion cells (figure 2(b)). However because the electrodes are actually closest to the nerve fiber layer axons overlying the ganglion cells, the possibility exists for the stimulating electrodes to activate these crossing axons from distal retinal ganglion cells before the local cell bodies (e.g. figure 2(b)). In humans and primates, the nerve fiber layer consists of unmyelinated axons 0.5–2 µm in thickness extending around the foveal region in a C-shaped series of decussations (Vrabec 1966). The nerve fiber layer can vary in thickness from around 34–45µm near the foveola to 8 µmin the periphery (Varma et al 2003, 1996) and is thinnest about the median raphe (Vrabec 1966).
In patients, stimulation by epiretinal stimulus array electrodes have generated electrophosphenes in their visual fields (Humayun et al 2003, Mahadevappa et al 2005). Ideally, stimulation by each electrode in the array on the retina surface should elicit a spatially adjacent electrophosphene in the patient’s ‘visual field’. However the current reported ‘visual fields’ of a single implanted subject of Humayun et al (2003) seem to be considerably larger than the epiretinal electrode array itself, generating the question whether surface electrodes can in some cases activate nerve fiber layer axons from more peripheral ganglion cells rather than the underlying local ganglion cell bodies (Greenberg et al (1999); see also Rattay and Resatz (2004)). In theory, smaller more densely packed electrode arrays close to the retinal surface might improve spatial resolution, however higher charge densities might be required to activate local ganglion cells. Modeling studies have suggested that line-shaped stimulation electrodes oriented parallel to the nerve fiber decussations could selectively activate local ganglion cells (Grumet et al 2000, Rattay and Rasatz 2004).
Epiretinal electrical stimulation of the retina can potentially activate multiple retinal pathways. In rabbit retina, stimulation studies of a large ganglion cell type with an epiretinal disc electrode have shown that a critical window exists between activation of the adjacent axon fiber bundles, the local ganglion cell bodies and the retinal network circuitry below (Jensen et al 2005a, 2005b). To single pulse stimulation, these response components have different latencies of action potentials, with the retinal network response being more delayed. Short duration pulses (0.1 ms) are more selective for direct activation of local ganglion cell bodies and not overlying axons (Jensen et al 2005a, Fried and Hsueh 2006). In rabbit retina, cathodic-first stimulation evoked lower spike thresholds than anodic stimulation (Jensen et al 2003, 2005a). In epiretinal prosthesis patients, electrode proximity to the retina also plays a role in determining electrophosphene current thresholds (Mahadevappa et al 2005). Future designs of epiretinal electrode arrays are slated to contain as many as a thousand electrodes closely apposed to the retina. Contingent on these arrays, given their smaller surface area, is the assumption that the localized electric field density will be sufficient to activate local ganglion cells directly below the electrodes while avoiding activation of more proximal axons crossing in the nerve fiber layer. Classically with electrical stimulation, large diameter axons are recruited before smaller axons. This is known as the ‘size principle’. Unmyelinated ganglion cell axons show varicosities inside the human eye, and this could also potentially affect axon recruitment thresholds to epiretinal electrical stimulation (Wang et al 2003).
Finally, there are limits to electrical stimulation of the retina using metal electrodes. Most epiretinal prostheses using metal electrodes rely on short charge-symmetric pulses on the order of milliseconds to excite the retinal network. This is because longer current pulses, similar to the natural response of retinal neurons to light, cause metal electrodes to degrade (Rose and Robblee 1990, Robblee et al 1983). Consequently, stimulation tends to be limited to biphasic charge-conserving short pulses. Most methods of electrical stimulation cannot distinguish between ON-and OFF-center ganglion cell types (but see Jensen and Rizzo (2006)). It is unclear at present if electrical stimulation can selectively stimulate different ganglion cell types. Different ganglion cell types have different natural firing patterns and spontaneous firing rates; however, they will all be stimulated simultaneously by electrical stimulation. New finer electrode designs may be able to better focus the electric field at the local neurons in the retinal network. None the less, any visual prosthesis that could provide even rudimentary spatial pattern vision would be a major advance for the severely blind patient.
6.3. Eye movements and image stabilization
To be able to see fine detail in the visual world with our fovea. we need to repeatedly shift our direction of gaze through eye movements. The globe of the eye is capable of turning at speeds of up to 900◦ s(Carpenter 1988). This presents severe challenges as to how to send electrical power and signals to prosthetic devices mounted on the surface of the eyeball. The outer surface of the eyeball or sclera, while tough from collagen fibrils, is quite thin in humans. Scleral thickness varies from 0.5 mm near the peripheral surgical limbus to 1 mm at the optic nerve head (Olsen et al 1998). Eye movements generated by the six extraocular muscles that surround the eye are of several different types. They include large fast excursions called ‘saccades’ of up to 100◦, smaller ‘smooth pursuit’ movements and fine ‘microsaccades’. Estimates of the daily rate of saccadic eye movements are task-dependent, and vary from 10 000 to 170 000 per day (Dr Jeff Pelz, personal communication, Schiller and Tehovnik (2002)). Seventy-five per cent or more of saccades during ambulatory movement or in tasks such as hand washing are under 15 ◦ s−1 (Pelz and Canosa 2001, Bahill et al 1975). To avoid these movements of the globe stressing connecting cables in retinal prostheses, current retinal stimulating prosthetic designs have proposed using devices with telemetry coils mounted to the globe itself, visible or IR light beams as intraocular power/signal sources, or multiple turns of flexible cable to reduce retinal tension (Humayun et al 2003, Javaheri et al 2006, Wickelgren 2006).
The spontaneous eye movements of visually impaired patients can be a significant problem for prosthesis designs. Adult-onset blind RP patients can exhibit continuous nystagmus or spontaneous eye movements (Ohm 1951, Leigh and Zee 1980, Kompf and Piper 1987, Mahadevappa et al 2005), and blindness from birth has been associated with an impaired vestibulo-ocular reflex and an inability to voluntarily initiate saccades (Leigh and Zee 1980). These patient eye movements cause the electrophosphene of a fixed position on the visual cortex/retina to apparently move. As first noted for cortical prostheses, eye movements have made it difficult to generate a ‘visual field’ map of the electrophosphenes evoked from adjacent electrodes (Brindley and Lewin 1968, Dobelle and Mladejovsky 1974, Schmidt et al 1996). Many visual prostheses currently rely on a camera mounted on eyeglasses for transmitting the visual signal sent to their stimulating electrode arrays (e.g. figure 1). These head mounted camera designs do not compensate for patient eye movements. This may require the prosthesis patient to learn additional forms of compensation (e.g. head scanning) in order to navigate the visual environment (e.g. Dobelle (2000)). The problem of remapping the moving visual scene is eliminated only in prosthetic designs that use a ‘camera’ mounted on the eye, or when eye movement tracking/correction is employed (e.g. Gekeler and Zrenner (2005)).
7. Lateral geniculate nucleus
The lateral geniculate nucleus (LGN) in the thalamus is the first visual area to receive synaptic input from the optic nerves. Its retinotopic organization makes it a potential target for visual prosthetic devices. The human LGN is a rounded structure composed of six concentric slightly bent layers of neurons, and is fairly small (approx. 6 mm × 5 mm). There is considerable variation between individuals in the lateral extent of each layer (Hickey and Guillery 1979, Andrews et al 1997). The lower two LGN layers receive input from magnocellular retinal ganglion cells, while the upper four LGN layers receive input from parvocellular cells, with each layer receiving exclusive input from either the ipsilateral or contralateral eye. Most studies on human LGN retinotopy have relied on non-invasive imaging methods particularly functional MRI (fMRI) which relies on the oxygen-dependent hemodynamic response of neural tissue. Studies in primate visual cortex indicate that this fMRI response is roughly correlated with the local field potential activity when both are measured simultaneously (Logothetis et al 2001). Functional MRI studies of the human LGN indicate that foveal vision is represented in the posterior and superior sections and is highly enlarged (Schneider et al 2004). Magnocellular portions of the geniculate input appeared more inferior and medial in location. From fMRI studies, a 3◦ retinal area of central vision is magnified anatomically in the lateral geniculate nucleus to occupy ∼60% of the LGN volume (figure 6, Schneider et al (2004)). This enlarged LGN foveal representation could facilitate stimulation of the central visual area by a prosthetic device. Electrical stimulation studies of human LGN have been reported by Nashold (1970) and Marg and Dierssen (1965). In some cases, subjects were able to perceive discrete colored dots; however, the surgical difficulty of the deep brain approach to the LGN has hampered prosthetic design. A visual prosthetic design projecting to the LGN has been proposed (Yagi et al 1999, 2005).
8. Visual cortex
The primary visual cortex, termed ‘V1’, ‘occipital’, or ‘striate cortex’ serves as the principal way station for visual processing in the brain, and has long been a target for visual prostheses (Marg 1991). Its surface is covered by protective meninges composed of an outer ‘dura mater’ of tough white fibrous and elastic tissues, a middle ‘arachnoid’ membrane, and a vascular ‘pia mater’ that covers the cortical surface. Human visual cortex receives synaptic input from the LGN through a prominent series of axonal fiber tracts termed ‘optic radiations’ that course to the occipital pole of the cerebral cortex and form part of the white matter below the cortical layers. Stimulation of these radiations can elicit electrophosphenes (Marg and Dierssen 1965).
8.1. Anatomical organization
The visual cortex is composed of ∼100 neuron types irregularly arranged in distinct laminae numbered 1–6 from the cortical surface (Born 2001) A radial section through human visual cortex is shown in figure 3(a). The axonal endings of LGN efferents synapse onto visual cortical neurons located in layers 4Cα and 4Cß. The receptive fields of neurons in these layers have a simple center-surround organization as originally defined by Hubel and Wiesel (1962). More distal layers contain pyramidal neurons that have ‘complex’ visual receptive fields and dendrites that span the cortical layers. Cells near the visual cortical surface have complex receptive field properties. Perhaps the most striking feature of the visual cortical surface is that it is highly organized by visual modality. Adjacent visual areas termed ‘hypercolumns’ receive LGN input segregated by right or left eye, stimulus orientation, and also by color in a complex spatial representation of the visual field.
Anatomical studies of human visual cortex using biocytin or DiI to label neurons have found extensive axonal collaterals between adjacent cortical regions (Burkhalter and Bernardo 1989, Kenan-Vaknin et al 1992, Burkhalter et al 1993). Activation of these axon collaterals by stimulus electrodes have the potential to spatially blur or cause multiple phosphene percepts (Brindley and Lewin 1968). Layer 1 is closest to the cortical surface. It is a largely aneuronal layer, and is composed of many axonal fibers that branch to adjacent local areas in visual cortex. In primates, many of these axons are projections from higher cortical areas such as V2, MT, inferotemporal cortex (Rockland and Knudsen 2000, Rockland et al 1994). Layers 2/3 termed the ‘supragranular layers’ contain many somata and dendrites of pyramidal cells. These cells have extensive axons projecting to extrastriate areas including V2, V3, V4, MT among others. The deeper cortical layers such as layers 4A, 4B and 4C (α, ß) termed ‘the granular layers’ have few axonal collaterals. Axons in layer 5/6 (infragranular layers) project back to the lateral geniculate nucleus. In addition, there is a series of extensive narrow vertical excitatory axonal connections from infragranular cortical layers to the supragranular layers and inhibitory feeback as well (for more details see Alonso (2002), Burkhalter et al (1993), Kenan-Vaknin et al (1992)). How these lateral and vertical feedback axonal pathways respond to electrical stimulation is unknown.
9. Visual cortex prostheses
9.1. An enlarged retinotopic representation
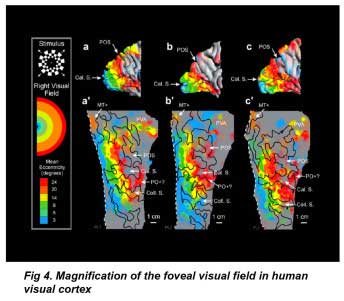
A significant advantage of the human visual cortex (VI) for prosthesis design is that the cortical surface area occupied by the central retinal visual field is highly magnified (figure 4). Visual cortex fMRI studies indicate that the foveal visual field occupies the posterior pole of the occipital lobe (DeYoe et al 1996). Using fMRI on seven subjects’ cortices, Dougherty et al (2003) found a 2◦ radius of central retinal visual field ∼1.0 mm2, occupied on average 2095 ± 638 mm2 in the visual cortex: a 2000-fold expansion. Conceivably, this would make the task of central visual field stimulation considerably easier, and one visual prosthetic device using platinum disc electrodes to stimulate the cortical surface was in clinical trials in Portugal (Kotler 2002). However, a portion of the central visual field lies in the calcarine sulcus, so not all of the visual field is surface accessible (figure 3(b)). There is also significant variation in the size of the visual cortex between individuals (Sauer 1983, Stensaas et al 1974, DeYoe et al 1996, Dougherty et al 2003) (figure 4). Two types of electrodes have been tested in visual cortex prostheses: surface and intracortical electrodes.
9.2. Cortical surface electrode arrays
Cortical surface stimulation with disc electrodes, such as those employed originally by Giles Brindley (Brindley and Lewin 1968) and the late William Dobelle (2000, 1996, 2005 obituary) have been tested in significant numbers of patients (see also Lee et al (2000a)). This type of cortical implant is the least invasive, beside the inherent risks associated with brain surgery, tissue traction and material biocompatibility. The initial studies of Brindley and Lewin (1968) used a single array of 80 silicon-insulated square Pt electrodes each 0.64 mm2 that were placed over the pial surface of the visual cortex. Each electrode was connected by wires to a single pedestal interface coil mounted on the skull. Electromagnetic coil induction was used to stimulate individual electrodes (Brindley 1982, Brindley and Lewin 1968). Later prosthesis designs increased the electrode number to 151 (Brindley 1982).
Using surface stimulation, high biphasic current pulse trains (in the 0.5–5 mA range) are necessary to elicit electrophosphenes, necessitating the use of large surface electrodes to avoid electrochemical degradation (e.g. Girvin et al (1979)). At threshold levels, patients typically describe stimulation-induced electrophosphenes in the central visual field as being single point-like or pea-sized bright stimuli seen at arm’s length. More peripherally located electrophosphenes have been reported as looking like small clouds (Brindley and Lewin 1968). Stronger levels of stimulation elicit electrophosphenes that can appear in multiple regions of the visual field perhaps due to activation of adjacent gyral regions of cortical surface or axon collaterals. High-level stimulation can in some cases elicit frontal headaches presumably due to activation of the meningial pain fibers (Brindley 1982), and in one case convulsions have been reported (Kotler 2002). Using surface stimulation electrodes, two-point discrimination of the cortex is poor and only distances of 3 mm can be resolved (Dobelle and Mladejovsky (1974), Dobelle et al (1974); see also Talalla et al (1974)). Brindley and Lewin (1968) estimated the resistivity of the cortical membrane as 390–500 cm.
When individual electrodes on the cortical surface are stimulated, a retinotopic map of electrophosphene locations in the visual field can be generated. The resulting map appears as a rough sector of some 20◦ of visual field, giving the patient a pie slice-like region for visual navigation (Brindley et al 1972, Dobelle 2000). However map construction is complicated by the fact that during voluntary eye movements, the electophosphenes move with the eyes, except during the vestibular reflex (Brindley and Lewin 1968). When mapped electrodes were stimulated simultaneously in order to generate a ‘square’ electrophosphene percept, the square became distorted indicating the electrophosphenes were not spatially independent (Dobelle et al 1974). Electrodes may also be ineffective in stimulating regions of the visual field that lie in the calcarine sulcus (e.g. figure 3(b)) or under blood vessels. In addition a ‘secondary dura’ has been reported to form around implanted surface electrode arrays (Brindley and Lewin 1968).
In the Dobelle group cortical prosthesis trials, the patient used a camera mounted to a pair of glasses for visual input (Dobelle 2000, Kottler 2002). The visual processing unit consisted of a belt-mounted computer and utilized an edge detection algorithm for analyzing the visual scene (Sobel 1970). The system was implanted originally in four blind patients (Dobelle 2000). Early studies of the interface Dobelle (2000) were limited to 64 electrodes stimulating one visual cortex. The Teflon-insulated electrode array was connected to the outside electronics through a pyrolytic carbon percutaneous pedestal connector (Klomp et al 1977, 1979). More recently, a group of 16 patients were reportedly implanted by Dobelle with bilateral 72 Pt disc electrode arrays in a clinical trial in Portugal in 2002. These trials received great media attention (Kotler 2002) including a video of an implanted individual driving a car around an empty parking lot. To date however, no peer-reviewed published clinical studies have documented patient visual performance or device stability. In addition, the higher stimulation currents required by surface electrode prostheses consume more power, making large batteries needed for portable use.
9.3. Intracortical electrode arrays
Intracortical microelectrode stimulation of the visual cortex has the advantage that significantly lower stimulus currents are needed to evoke electrophosphenes compared to surface electrodes (typically 10–20 µA versus 1–10 mA) (e.g. Ronner (1982), Tehovnik (1996)). Intracortical stimulation is currently being studied by the labs of Troyk and Normann. Typical experiments implant long needle-type electrode arrays (5–150 electrodes) in primate visual cortex (e.g. Bradley et al (2005)). However the impalement of visual cortex by intracortical electrodes can in some cases injure the brain, cause seizures, or even damage the microelectrodes themselves, and special electrode inserters have been devised (Normann et al 1999). In addition, reactive gliosis can in some cases form capsules around implanted electrodes in felines (Schmidt et al 1993,Liu et al 1999).
A clinical trial of an intracortical electrode array implanted to passively record neural activity in a paralyzed patient’s motor cortex is currently ongoing (Hochberg et al 2005 abstract). This microarray, termed the ‘Utah array’ is currently composed of a 10 × 10 array of Pt-tipped silicon microneedles insulated with silicon nitride (Normann et al 1999, Warren et al 2001). The Utah array is connected by a polyimide cable to a cranial pedestal connector to the skull. Array electrode experiments in primate motor cortex (MI) that indicate arm movements can be predicted from the recorded neural firing patterns (Serruya et al 2002). However it is unclear whether these microarray electrodes will be stable enough to pass the larger currents required for stimulation of cortical neurons (see also Warren and Normann (2005)).
Few studies have used intracortical electrodes for stimulation in the human visual cortex (Shakhnovich et al 1982,Bak et al 1990, Schmidt et al 1996). Thirtyeight parylene-insulated iridium electrodes were chronically implanted in pairs in a 42 year old blind patient (Schmidt et al 1996). Electrode spacings tested were 250, 500 and 750 µm. Electrophosphene thresholds were complicated by the subject’s perception of spontaneous phosphenes (Bak et al 1990). Shorter pulse width stimuli (200 µS) aided electrophosphene perception. Electrophosphenes elicited by pulse trains longer than 1 s usually disappeared. Although data were limited, electrophosphenes elicited by two electrodes spaced 250 or 500 µm apart were often reported as being in the same visual field location; implying inter-electrode spacings of 500 µm may be required (Bak et al 1990).
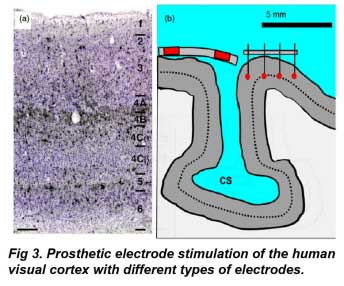 In Fig 3a, Neuronal layers of the adult human visual cortex in radial section. Neurons in black are immunostained with an antibody (Cat-301) that labels cells thought to be involved in the magnocellular ganglion cell pathway (black stain), while other neurons are counterstained in blue (Nissle stain). Note layer 1, the uppermost layer near epidural disc electrodes, is largely devoid of neurons. Scale bar: 250 µ From Preuss and Coleman (2003) with permission. Fig 3b shows a visual cortex model implanted with epidural (left) and intracortical (right) stimulating electrodes. Red indicates conductive regions. Dotted line shows relative level of layer 4 geniculate efferents to cortex. A portion of the central visual field lies in the calcarine sulcus (CS), which is difficult to contact by current electrode designs. Fixed length intracortical electrodes may not stimulate neurons in the same layer due to variation in cortical curvature. CS: calcarine sulcus. Adapted from a section in figure 1, Stensaas et al (1974).
In Fig 3a, Neuronal layers of the adult human visual cortex in radial section. Neurons in black are immunostained with an antibody (Cat-301) that labels cells thought to be involved in the magnocellular ganglion cell pathway (black stain), while other neurons are counterstained in blue (Nissle stain). Note layer 1, the uppermost layer near epidural disc electrodes, is largely devoid of neurons. Scale bar: 250 µ From Preuss and Coleman (2003) with permission. Fig 3b shows a visual cortex model implanted with epidural (left) and intracortical (right) stimulating electrodes. Red indicates conductive regions. Dotted line shows relative level of layer 4 geniculate efferents to cortex. A portion of the central visual field lies in the calcarine sulcus (CS), which is difficult to contact by current electrode designs. Fixed length intracortical electrodes may not stimulate neurons in the same layer due to variation in cortical curvature. CS: calcarine sulcus. Adapted from a section in figure 1, Stensaas et al (1974).
9.4. Critical issues for visual cortical stimulation
Given the extensive cortical magnification of the foveal visual field, a critical issue in intracortical electrode design is improving the two-point discrimination between stimulating electrodes. To date, we have little data on what might be the optimal depth(s) or design for intracortical stimulation electrodes in humans. The human visual cortex is about 2.5– 3 mm in thickness (Leuba and Kraftsik 1994, Barbier et al 2002, Fischl and Dale 2000, Salat et al 2004). Although the axons of geniculate fibers lie deep to the cortical surface, electrode activation of more shallow axon feedback collaterals found in the intrinsic organization of the visual cortex itself could enlarge the phosphene. How electrophosphene size varies with cortical depth is unknown in humans. A recent study in primates by DeYoe et al (2005) has suggested electrophosphene detection threshold sensitivity in visual cortex is broadly distributed between cortical layers; peaking in layers 3–4B, with a weaker second peak in layer 5. There may also be variations in the optimal phosphene depth depending on cortical layer orientation to the fixed array electrode (figure 3(b)). How intracortical electrodes can be constructed to selectively stimulate adjacent cortical regions situated in cortical sulci is currently unclear. Finally, it has been known in primates for many years that pulse train stimulation with intracortical electrodes in the µA range can evoke eye saccades to the presumed electrophosphene, unless the subject is actively fixating on a target (Doty 1965, Talalla et al 1974, Schiller 1977, Keating and Gooley1983, 1988). In primates, the current-depth profile for evoking saccade thresholds is lowest near the layer 5/6 border and highest near the dural surface (Tehovnik et al 2003).
10. The visual prosthetic implant and the brain vasculature
A final concern for prosthetic device design is the integration of the device with the vasculature of the brain. Like most brain neurons, visual system neurons receive extensive blood flow and are highly sensitive to anoxia. The design and placement of a prosthetic device so that it does not significantly occlude blood flow/tissue oxygenation is an important consideration for stimulating the remaining neural neurons without inducing additional damage.
10.1. The retinal vasculature
The retina is one of the most oxygen-consuming structures in the human body. The bulk of the oxygenation of the retina is delivered through blood flow in the posterior choroidal capillaries located behind the retina. These choroidal vessels form an extensive capillary layer mat directly adjacent to the photoreceptors termed the ‘choriocapillaris’. The choriocapillaris receives the greatest percentage of retinal blood flow (65–85%) (Henkind et al 1979) and is vital for the maintenance of the photoreceptor layer. A lesser degree of blood flow occurs inside the retina itself via the central retinal artery. These blood vessels service inner retinal layer neurons such as ganglion and amacrine cells (see also Alm and Bill (1973)). The retinal artery capillaries end at the photoreceptor terminals. Thus the bulk of blood flow occurs behind the photoreceptor cells, so as to not obscure the retinal image. Between the choriocapillaris and the retinal artery capillary beds, exists an avascular region some 130 µm in width that depends on diffusion of oxygen from the two capillary beds (Cioffi et al 2003). A consequence of this arrangement is that the maximum oxygen tension in a primate eye, some 85– 100 mm Hg declines with distance away from the choriocapillaris into the inner retina. The oxygen tension of the inner retinal circulation is weaker and reaches a peak of 30 mm Hg.
Subretinal prostheses can potentially occlude choriocapillaris oxygen diffusion. Studies with impermeable microphotodiode implants in the subretinal space of rats and cats show some thinning of the adjacent photoreceptors, and the bipolar and amacrine cells in the inner nuclear layer (Chow et al 2001, 2002,Volker et al 2004); however, implants in pigs showed less degeneration of the inner retina (Zrenner et al 1999). For subretinal implants in humans, the central foveal region (500–600 µm diam.) lacks retinal capillaries entirely and is dependent on oxygen diffusion from the choriocapillaris bed (Laatinainen and Larinkari 1977).
10.2. The visual cortex vasculature
The visual cortex vasculature presents different issues for prosthetic designs. It is well known that the visual cortex moves slightly due to the pulsatile nature of arterial blood flow in the brain (Glover and Lee 1995). Significant cortical movement pulsations can also occur during coughing or due to head impact (Maier et al 1983, Schmidt et al 1996, Goldberg et al 2005). A consequence of this issue is that visual prosthetic devices implanted into cortical tissue are subjected to continual flexing which could potentially fatigue fine coatings used to inject current, passivation layers used for insulation, or connections to the skull.
The pial surface of the cortical brain has extensive blood vessels that can be damaged in implanting intracortical electrode arrays. Capillary beds are also found in all cortical layers with the largest numbers of capillaries in layer IVC (Bell and Ball 1985). Close post-operative management of implanted subjects may be needed to avoid seizures, infection, blood hemorrhages, or strokes (Bradley et al 2005). However, experiments in primates and cats with implanting intracortical stimulating electrodes by Liu et al (1999), Warren and Normann (2005) and Troyk et al (2003) show that tissue insult with electrode insertion in most cases attenuates with time.
11. Future visual prostheses
Although current visual prostheses rely on electrical stimulation to excite neurons, researchers are trying more biologically compatible methods of neuron stimulation. These methods include using natural brain neurotransmitters/analogs, ion channels and neuroattractive surfaces to improve signal transmission across the ‘machine– neuron’ interface.
11.1. Neurotransmitter-releasing visual prostheses
Glutamate is the major neurotransmitter released by many neurons in the retina and visual cortex including retinal photoreceptors and geniculate efferents. In the retina, glutamate is normally stored in the endings of rod and cone photoreceptors as synaptic vesicles (∼100 mM) and released onto bipolar cell dendrites. In theory, glutamate released onto bipolar cells would depolarize OFF-bipolar cells, while simultaneously hyperpolarizing ON-bipolar cells. If a glutamate-releasing prosthesis could be placed at the level of the missing photoreceptor endings in RP patients, communication with the remaining bipolar cells could propagate the visual signal to ganglion cells. Two glutamatereleasing prosthetic designs are currently being proposed; one using optical waveguide uncaging of neurotransmitter near retinal neurons (Safadi et al 2003), and another using microfluidic neurotransmitter release through miniature orifices (Peterman et al 2003a, 2004). Neurotransmitterreleasing prosthetic designs have also been tested in the cerebral cortex by Cheung et al (2003), and also in the guinea pig superior colliculus (Rathnasingham et al 2004,Wise et al 2004, Chen et al 1997).
There are also potential problems with this form of more natural neural stimulation. The glutamate-releasing prosthetic device needs to be located extremely close to the glutamate receptors on the dendrites of the postsynaptic bipolar cell targets. Externally applied glutamate, if applied more distally, is rapidly uptaken from the extracellular space by several different glutamate transporter systems present in Muller cells, photoreceptors and some bipolar cells (Grewer and Rauen 2005, Amara and Fontana 2002). Conceivably, this loss could be alleviated by prosthetic devices that ‘tether’ a neurotransmitter analog as a movable ligand near the receptor (Vu et al 2005, Nehilla et al 2004). Because the normal resting potential of photoreceptor cells in the dark is partially depolarized, some sustained glutamate release by photoreceptor endings is normally present (Heidelberger et al 2005). Bipolar and ganglion cells are also partially depolarized due to resting glutamate release (e.g. Cohen and Miller (1994), Cohen (1998, 2001)). Glutamate diffusion could be limited by growing retinal neurons into small orifices (e.g. Peterman et al (2003b)). It is also unclear how a neurotransmitter releasing prosthesis would be refilled. Nonetheless, this neurotransmitter has the greatest potential for duplicating natural vision in an ocular prosthetic device.
11.2. Neural interfaces using light-or mechanically-activated ion channels
It is possible to engineer ion channel interfaces for stimulating neurons that can be gated directly by light. Qiu et al (2005) transfected mammalian cells with an endogenous mammalian opsin, melanopsin which caused them to elicit slow lightinduced depolarizations. Nagel et al (2003) cloned an ion channel termed ‘channel rhodopsin-2’ from the green algae chlamydomonas reinhardtii that was gated directly by light. Upon illumination, a non-selective calcium-permeable cation channel opened causing rapid depolarizations of the membrane. Several groups have transfected cultured hippocampal and retinal neurons with channel rhodopsin-2 and show they are able to gate neuron firing with light; in some cases with near millisecond time control (Banghart et al 2004, Bi et al 2006, Boyden et al 2005). Finally mechanosensitive channels such as the stretch-activated MscL channel in bacteria have been genetically altered to be gated by light directly (e.g. Kocer et al (2005)).
11.3. Electrically stimulated cultured neural network interfaces
Neurons can also be stimulated by being grown on silicon substrates with electrical pads. (Matsuzawa et al 1994, Fromherz 2003). A ‘bio-hybrid’ prosthesis has been proposed where a silicon chip would stimulate cultured retinal ganglion cells which would then form synaptic projections to a blind patient’s LGN neurons (Yagi et al 1999, 2005). In the neonatal mouse, retinal neurons will grow into sculptured devices and can be electrically stimulated with current (Peterman et al 2003b, Leng et al 2004). Whether a similar process can be elicited in adult retinal tissue is unclear at present.
12. Conclusions and common issues to visual prostheses
In discussing the status and development of prosthetic devices for the blind, it is instructive to recall the history of cochlear prosthetic implants for the deaf. The cochlea is a one-dimensional neural structure which resolves sound frequency. The human auditory nerve contains ∼30 000 axons which cochlear implants currently stimulate with 8– 22 electrodes for a ratio of ∼1:3000 (ten electrodes). The first cochlear implant was introduced in 1972 over 30 years ago. Cochlear implants had a long history of clinical trial failures, before gaining acceptance (Blume 1999). Initially, patient acceptance of cochlear implants was poor; however with continued use, improved speech processors and electrode arrays the remarkable plasticity of the brain to adapt to new representations of the external auditory environment have made cochlear implants a success.
Visual prosthetic devices may evolve along a similar development path. About a million retinal optic nerve fibers innervate the brain. Future visual prostheses are slated to contain ∼250 electrodes resulting in a similar axon to electrode ratio as current cochlear prostheses (∼1:4000) to stimulate a two-dimensional visual structure. Given the adaptability of the human brain, it seems possible visual prostheses could enable the visually impaired patient to gain some functional vision. However like cochlear implants, the visual prosthetic world is likely to differ considerably from our normal visual percepts (Merabet et al 2005). Several different designs may be of clinical utility, as not all patients will have similar lesions or diseases of the visual system.
12.1. Common biological issues
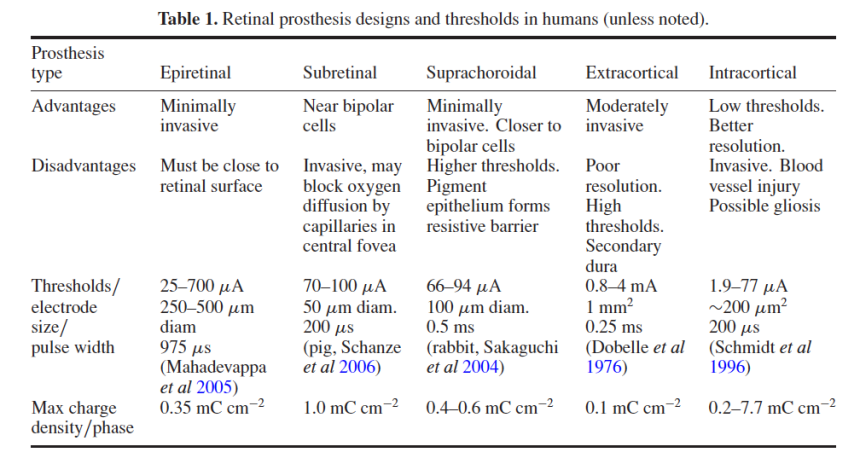
Visual prosthetic devices must display some programmable adaptation to the patient’s biology. Because the visual abilities/disease state of each implant candidate is different, the placement of stimulation electrodes near visual system neurons will not always achieve the same result. Each implant design has different advantages and electrical phosphene thresholds (table 1). To compensate for two-dimensional variations in retinal and cortical neural tissue, stimulation arrays may require remapping and electrodes may need multiple stimulation zones to optimize a patient’s visual phosphene fields. Stimulation patterns may need to be compensated for patient eye movements by mechanisms such as eye tracking to avoid movement of the perceived ‘visual phosphene fields’.
New stimulation electrode designs are needed to optimally replicate the natural biological function of the visual pathways and improve the prosthesis–neuron interface. Selective stimulation of ON-and OFF-center retinal ganglion cell pathways may be needed to improve contrast perception. To improve two-point visual discrimination, better electrode designs are needed to stimulate local neurons. Blurring of the visual scene by activation of axonal fibers of passage such as found in the retinal nerve fiber layer or collateral axons in the visual cortex needs to be avoided.
Finally, most visual degenerative diseases affect the retina. Whether a stimulating prosthetic array inserted in the patient’s eye can adequately maintain the functional integrity of a diseased retina is unclear at present. In contrast, the visual cortex is unaffected by most diseases causing blindness. However, the ease of insertion and monitoring retinal prostheses may offset the relative risks of seizures, gliosis, hematomas and meningitis associated with implantation of cortical stimulation arrays (e.g. Lee et al (2000b)). If visual prostheses follow the development timeline for cochlear implants, durability, long-term patient use, learning and training will be essential for these devices to gain acceptance. In addition, more biologically relevant encoding strategies and designs of the neuron-prosthetic stimulation interface will enhance patient benefit.
References
Acland G, Aguirre G, Ray J, Zhang Q, Aleman T, Cideciyan A V, Pearce-Kelling S E, Anand V, Zeng Y, Maguire A M, Jacobson S G, Hauswirth W W and Bennett J 2001 Gene therapy restores vision in a canine model of childhood blindness Nat. Genet. 28 92–5
Alm A and Bill A 1973 Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labeled microspheres including flow determinations in brain and some other tissues Exp. Eye Res. 15 15–29
Alonso J 2002 Neural connections and receptive field properties in the primary visual cortex Neuroscientist 8 443–56 Amara S G and Fontana A C 2002 Excitatory amino acid transporters: keeping up with glutamate Neurochem. Int. 41 313–8
Andrews T J, Halpern S D and Purves D 1997 Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract J. Neurosci. 17 2859–68
Bahill A T, Adler D and Stark L 1975 Most naturally occurring human saccades have magnitudes of 15 degrees or less Invest. Ophthalmol. 14 468–9
Bak M, Girvin J P, Hambrecht F T, Kufta C V, Loeb G E and Schmidt E M 1990 Visual sensation produced by intracortical microstimulation of the human occipital cortex Med. Biol. Eng. Comput. 28 257–9
Banghart M, Borges K, Isacoff E, Trauner D and Kramer R H 2004 Light-activated ion channels for remote control of neuronal firing Nat. Neurosci. 7 1381–6
Barbier E L, Marrett S, Danek A, Vortmeyer A, van Gelderen P, Duyn J, Bandettini P, Grafman J and Koretsky A P 2002 Imaging cortical anatomy by high-resolution MR at 3.0T: detection of the stripe of Gennari in visual area 17 Magn. Reson. Med. 48 735–8
Bell M A and Ball M J 1985 Laminar variation in the microvascular architecture of normal human visual cortex (area 17) Brain Res. 335 139–43
Berson E L, Rosner B, Weigel-DiFranco C, Dryja T P and Sandberg M A 2002 Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations Invest. Ophthalmol. Vis. Sci. 43 3027–36
Bi A, Cui J, Ma Y, Olshevskaya E, Pu M, Dizhoor A and Pan Z H 2006 Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration Neuron 50 23–33
Blume S S 1999 Histories of cochlear implantation Soc.Sci.Med. 49 1257–68
Born R T 2001 Visual processing: parallel-er and parallel-er Curr. Biol. 11 R566–8
Boycott B B and W¨assle H 1991 Morphological Classification of Bipolar Cells of the Primate Retina Eur. J. Neurosci. 3 1069–88
Boyden E S, Zhang F, Bamberg E, Nagel G and Deisseroth K 2005 Millisecond-timescale, genetically targeted optical control of neural activity Nat. Neurosci. 8 1263–8
Bradley D C, Troyk P R, Berg J A, Bak M, Cogan S, Erickson R, Kufta C, Mascaro M, McCreery D, Schmidt E M, Towle V L and Xu H 2005 Visuotopic mapping through a multichannel stimulating implant in primate V1 J. Neurophysiol. 93 1659–70
Brindley G S 1955 The site of electrical excitation of the human eye. Physiol. 127 189–200 Brindley G S 1982 Effects of electrical stimulation of the visual cortex Hum. Neurobiol. 1 281–3
Brindley G S, Donaldson P E, Falconer M A and Rushton D N 1972 The extent of the region of occipital cortex that when stimulated gives phosphenes fixed in the visual field J. Physiol. 225 57P-58P
Brindley G S and Lewin W S 1968 The sensations produced by electrical stimulation of the visual cortex J. Physiol. 196 479–93
Brummer S B and Turner M J 1977 Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes IEEE Trans. Biomed. Eng. 24 59–63
Burkhalter A, Bernardo K L and Charles V 1993 Development of local circuits in human visual cortex J. Neurosci. 13 1916–31
Burkhalter A and Bernardo K L 1989 Organization of corticocortical connections in human visual cortex Proc. Natl Acad. Sci. USA 86 1071–5
Burkhardt D A and Fahey P K 1998 Contrast enhancement and distributed encoding by bipolar cells in the retina. Neurophysiol. 80 1070–81
Cao W, Li F, Steinberg R H and Lavail M M 2001 Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the rat retina Exp. Eye Res. 72 591–604
Cao W, Wen R, Li F, Lavail M M and Steinberg R H 1997 Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina Exp. Eye Res. 65 241–8
Carpenter R H S 1988 Saccades Movements of the Eyes (London: Pion Press) chapter 4 Cheung K C, Djupsund K, Dan Y and Lee L P 2003 Implantable multichannel electrode array based on SOI technology. Microelectromech. Syst. 12 179–84
Chen J, Wise K D, Hetke J F and Bledsoe S C Jr 1997 A multichannel neural probe for selective chemical delivery at the cellular level IEEE Trans. Biomed. Eng. 44 760–9
Chow A and Chow V 1997 Subretinal electrical stimulation of the rabbit retina Neurosci. Lett. 225 13–6
Chow A Y, Chow V Y, Packo K H, Pollack J S, Peyman G A and Schuchard R 2004 The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa Arch. Ophthalmol. 122 460–9
Chow A Y, Pardue M T, Chow V Y, Peyman G A, Liang C, Perlman J I and Peachey N S 2001 Implantation of silicon chip microphotodiode arrays into the cat subretinal space IEEE Trans. Neural Syst. Rehabil. Eng. 9 86–95
Chow A Y, Pardue M T, Perlman J I, Ball S L, Chow V Y, Hetling J R, Peyman G A, Liang C, Stubbs E B Jr and Peachey N S 2002 Subretinal implantation of semiconductor-based photodiodes: durability of novel implant designs J. Rehabil. Res. Dev. 39 313–21
Chowdhury V, Morley J W and Coroneo M T 2005 Stimulation of the retina with a multielectrode extraocular visual prosthesis ANZ J. Surg. 75 697–704
Cioffi G, Granstam E and Alm A 2003 Ocular circulation Adler’s Physiology of the Eye 10th edn, ed P Kaufman and A Alm (St Louis, MO: Mosby) chapter 33
Cohen E D 1998 Interactions of inhibition and excitation in the light-evoked currents of X-type retinal ganglion cells. Neurophysiol. 80 2975–90
Cohen E D 2001 Synaptic mechanisms shaping the light-response in retinal ganglion cells Prog. Brain Res. 131 215–28
Cohen E D and Miller R F 1994 The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells Vis. Neurosci. 11 317–32
Curcio C A and Allen K A 1990 Topography of ganglion cells in human retina J. Comp. Neurol. 300 5–25
Curcio C A, Sloan K R, Kalina R E and Hendrickson A E 1990 Human photoreceptor topography J. Comp. Neurol. 292 497–523
Dacey D M, Liao H W, Peterson B B, Robinson F R, Smith V C, Pokorny J, Yau K W and Gamlin P D 2005 Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN Nature 433 749–54
Dacey D M and Packer O S 2003 Colour coding in the primate retina: diverse cell types and cone-specific circuitry Curr. Opin. Neurobiol. 13 421–7
Delbeke J, Oozeer M and Veraart C 2003 Position, size and luminosity of phosphenes generated by direct optic nerve stimulation Vis. Res. 43 1091–102
Delbeke J, Wanet-Defalque M C, Gerard B, Troosters M, Michaux G and Veraart C 2002 The microsystems based visual prosthesis for optic nerve stimulation Artif. Organs 26 232–4
DeYoe E A, Carman G J, Bandettini P, Glickman S, Wieser J, Cox R, Miller D and Neitz J 1996 Mapping striate and extrastriate visual areas in human cerebral cortex Proc. Natl Acad. Sci. USA 93 2382–6
DeYoe E A, Lewine J D and Doty R W 2005 Laminar variation in threshold for detection of electrical excitation of striate cortex by macaques J. Neurophysiol. 94 3443–50
Dobelle W H 2000 Artificial vision for the blind by connecting a television camera to the visual cortex ASAIO J. 46 1–7
Dobelle W H 2005 Obituary William H. Dobelle MD, 1941–2004; ASAIO Member, 1970-Present ASAIO J. 51 1–3
Dobelle W H and Mladejovsky W G 1974 Phosphenes produced by electrical stimulation of human occipital cortex and their application to the development of a prosthesis for the blind. Physiol. 243 553–76
Dobelle W H, Mladejovsky M G, Evans J R, Roberts T S and Girvin J P 1976 ‘Braille’ reading by a blind volunteer by visual cortex stimulation Nature 259 111–2
Dobelle W H, Mladejovsky M G and Girvin J P 1974 Artificial vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis Science 183 440–4
Doty R W 1965 Conditioned reflexes elicited by electrical stimulation of the brain in macaques J. Neurophysiol. 28 623–40
Dougherty R F, Koch V M, Brewer A A, Fischer B, Modersitzki J and Wandell B A 2003 Visual field representations and locations of visual areas V1/2/3 in human visual cortex. Vis. 3 586–98
Drasdo N and Fowler C W 1974 Non-linear projection of the retinal image in a wide-angle schematic eye Br. J. Ophthalmol. 8 709–14
Fariss R N, Li Z Y and Milam A H 2000 Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa Am. J. Ophthalmol. 129 215–23
Feucht M, Laube T, Bornfeld N, Walter P, Velikay-Parel M, Hornig R and Richard G 2005 Entwicklung einer epiretinalen Prothese zur Stimulation der humanen Netzhaut. Ophthalmol. 102 688–91
¨Reizung des Schapparates Arch. Psychiatr. Nevenkr. 26 867–85 (Cited in Brindley (1955))
Fischl B and Dale A M 2000 Measuring the thickness of the human cerebral cortex from magnetic resonance images Proc. Natl Acad. Sci. USA 97 11050–5
Fishman G A 1978 Retinitis pigmentosa Vis. Loss Arch. Ophthalmol. 96 1185–8
Fromherz P 2003 Neuroelectronic interfacing: semiconductor chips with ion channels, nerve cells and brain Nanoelectronics and Information Technology ed R Waser (Berlin: Wiley-VCH) chapter 32, pp 781–810
Fried S I, Hsueh H A and Werblin F S 2006 A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation J. Neurophysiol. 95 970–8
Gekeler F, Kobuch K, Schwahn H N, Stett A, Shinoda K and Zrenner E 2004 Subretinal electrical stimulation of the rabbit retina with acutely implanted electrode arrays Graefes Arch. Clin. Exp. Ophthalmol. 242 587–96
Gekeler F and Zrenner E 2005 Stand des subretinalen Implantatprojekts Eine Ubersicht. Ophthalmol. 102 941–9
Finklestein L 1894 Uber optische Ph¨anomeme bei electrischer Gerding H 2007 A new approach towards a minimal invasive retina implant J. Neural Eng. 4 S30–7
Girvin J P, Evans J R, Dobelle W H, Mladejovsky M G, Henderson D C, Abramov I, Gordon J and Turkel J 1979 Electrical stimulation of human visual cortex: the effect of stimulus parameters on phosphene threshold Sens. Process. 3 66–81
Glover G H and Lee A T 1995 Motion artifacts in fMRI: comparison of 2DFT with PR and spiral scan methods Magn. Reson. Med. 33 624–35
Goldberg C S, Antonyshyn O, Midha R and Fialkov J A 2005 Measuring pulsatile forces on the human cranium J. Craniofac. Surg. 16 134–9
Granit R and Helme T 1939 Changes in retinal excitability due to polarization and some observations on the relation between the processes in retina and nerve J. Neurophysiol. 2 556–65
Greenberg R, Velte T, Humayun M, Scarlatis G and de Juan E Jr 1999 A computational model of electrical stimulation of the retinal ganglion cell IEEE Trans. Biomed. Eng. 46 505–14
Grewer C and Rauen T 2005 Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling J. Membr. Biol. 203 1–20
Griffin D R, Hubbard R and Wald G 1947 The sensitivity of the eye to infrared radiation J. Opt. Soc. Am. 37 546–54
Grover S, Fishman G, Anderson R, Tozatti M, Heckenlively J, Weleber R, Edwards A and Brown J Jr 1999 Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older Ophthalmology 106 1780–5
Grover S, Fishman G and Brown J Jr 1998 Patterns of visual field progression in patients with retinitis pigmentosa Ophthalmology 105 1069–75
Grumet A, Wyatt J Jr and Rizzo J 2000 Multi-electrode stimulation and recording in the isolated retina J. Neurosci. Methods 101 31–42
Heidelberger R, Thoreson W B and Witkovsky P 2005 Synaptic transmission at retinal ribbon synapses Prog.Retin.Eye Res. 24 682–720
Hendrickson A E and Yuodelis C 1984 The morphological development of the human fovea Ophthalmology 91 603–12
Henkind P, Hansen R I and Szalay J 1979 Ocular circulation Physiology of the Human Eye and Visual System ed R E Records (New York: Harper and Row) pp 98–155
Hessburg P C and Rizzo J F III 2007 The Eye and the Chip. World Congress on Artificial Vision 2006 J. Neural Eng. 4 (1) (Editorial)
Hetling J R and Baig-Silva M S 2004 Neural prostheses for vision: designing a functional interface with retinal neurons Neurol. Res. 26 21–34
Heynen H and van Norren D 1985 Origin of the electroretinogram in the intact macaque eye–II: current source–density analysis Vis. Res. 25 709–15
Hickey T L and Guillery R W 1979 Variability of laminar patterns in the human lateral geniculate nucleus J. Comp. Neurol. 183 221–46
Hochberg L, Mukand J, Polykoff G, Friehs G and Donoghue J 2005 Braingate neuromotor prosthesis: nature and use of neural control signals Soc. Neurosci. 35 abstract 520.17
Hornig R and Richard G 2006 First clinical experience with a 50 electrode retinal implant The Eye and the Chip: Proc. World Congr. on Artificial Vision (Detroit, 15–17 June 2006)
Hubel D and Wiesel T 1962 Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex J. Physiol. 160 106–54
Humayun M S, de Juan E Jr, Dagnelie G, Greenberg R J, Propst R H and Phillips D H 1996 Visual perception elicited by electrical stimulation of retina in blind humans Arch. Ophthalmol. 114 40–6
Humayun M S, Prince M, de Juan E Jr, Barron Y, Moskowitz M, Klock I B and Milam A H 1999a Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa Invest. Ophthalmol. Vis. Sci. 40 143–8
Humayun M, de Juan E Jr, Weiland J, Dagnelie G, Katona S, Greenberg R and Suzuki S 1999b Pattern electrical stimulation of the human retina Vis. Res. 39 2569–76
Humayun M S et al 2003 Visual perception in a blind subject with a chronic microelectronic retinal prosthesis Vis. Res. 43 2573–81
Jacobson S G, Knighton R W and Levene R M 1985 Dark-and light-adapted visual evoked cortical potentials in retinitis pigmentosa Doc. Ophthalmol. 60 189–96
Javaheri M, Hahn D, Lakhanpal R, Weiland J and Humayun M 2006 Retinal prostheses for the blind Ann. Acad. Med. Singapore 35 137–44
Jensen R J, Rizzo J F III, Ziv O R, Grumet A and Wyatt J 2003 Thresholds for activation of rabbit retinal ganglion cells with an ultrafine, extracellular microelectrode Invest. Ophthalmol. Vis. Sci. 44 3533–43
Jensen R J, Ziv O R and Rizzo J F III 2005a Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes Invest. Ophthalmol. Vis. Sci. 46 1486–96
Jensen R J, Ziv O R and Rizzo J F 2005b Responses of rabbit retinal ganglion cells to electrical stimulation with an epiretinal electrode J. Neural Eng. 2 S16–21
Jensen R J and Rizzo J F 2006 Thresholds for activation of rabbit retinal ganglion cells with a subretinal electrode Exp. Eye Res. 83 367–73
Jones B W, Watt C B, Frederick J M, Baehr W, Chen C K, Levine E M, Milam A H, Lavail M M and Marc R E 2003 Retinal remodeling triggered by photoreceptor degenerations. Comp. Neurol. 464 1–16
Kanda H, Morimoto T, Fujikado T, Tano Y, Fukuda Y and Sawai H 2004 electrophysiological studies of the feasibility of suprachoroidal-transretinal stimulation for artificial vision in normal and RCS rats Invest. Ophthalmol. Vis. Sci. 45 560–6
Karwoski C and Xu X 1999 Current source-density analysis of light-evoked field potentials in rabbit retina Vis. Neurosci. 16 369–77
Keating E, Gooley S, Pratt S and Kelsey J 1983 Removing the superior colliculus silences eye movements normally evoked from stimulation of the parietal and occipital eye fields Brain Res. 269 145–8
Keating E G and Gooley S G 1988 Disconnection of parietal and occipital access to the saccadic oculomotor system Exp. Brain Res. 70 385–98
Kenan-Vaknin G, Ouaknine G E, Razon N and Malach R 1992 Organization of layers II–III connections in human visual cortex revealed by in vitro injections of biocytin Brain Res. 594 339–42
Kocer A, Walko M, Meijberg W and Feringa B L 2005 A light-actuated nanovalve derived from a channel protein Science 309 755–8
Klomp G F, Womack M V and Dobelle W H 1977 Fabrication of large arrays of cortical electrodes for use in man J. Biomed. Mater. Res. 11 347–64
Klomp G F, Womack M III and Dobelle W H 1979 Percutaneous connections in man Trans. Am. Soc. Artif. Int. Organs 25 1–7
Kolb H, Linberg K A and Fisher S K 1992 Neurons of the human retina: A Golgi study J. Comp. Neurol. 31 147–87
Kompf D and Piper H F 1987 Eye movements and vestibulo-ocular reflex in the blind J. Neurol. 234 337–41
Kotler S 2002 A half century of artificial-sight research has succeeded. And now this blind man can see Wired Magazine 10/09 Sept.
Laatinainen L and Larinkari J 1977 Capillary-free area of the fovea with advancing age Invest. Ophthalmol. Vis. Sci. 16 1154–7
Lee HW, Hong S B,Seo D W, TaeW S andHong SC 2000a Mapping of functional organization in human visual cortex: electrical cortical stimulation Neurology 54 849–54
Lee W S, LeeJK, Lee SA, KangJ K andKo T S 2000b Complications and results of subdural grid electrode implantation in epilepsy surgery Surg. Neurol. 54 346–51
Leigh R J and Zee R 1980 Eye movements of the blind Invest. Ophthalmol. Vis. Sci. 19 328–31
Leng T, Wu P, Mehenti N Z, Bent S F, Marmor M F, Blumenkranz M S and Fishman H A 2004 Directed retinal nerve cell growth for use in a retinal prosthesis interface Invest. Ophthalmol. Vis. Sci. 45 4132–7
Leuba G and Kraftsik R 1994 Changes in volume, surface estimate, three-dimensional shape and total number of neurons of the human primary visual cortex from midgestation until old age Anat. Embryol. (Berlin) 190 351–66
Li Z Y, Kljavin I J and Milam A H 1995 Rod photoreceptor neurite sprouting in retinitis pigmentosa J. Neurosci. 15 5429–38
Liu X, McCreery D B, Carter R R, Bullara L A, Yuen T and Agnew W F 1999 Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes IEEE Trans. Rehabil. Eng. 7 315–26
Logothetis N K, Pauls J, Augath M, Trinath T and Oeltermann A 2001 Neurophysiological investigation of the basis of the fMRI signal Nature 412 150–7
MacLaren R, Pearson R, MacNeil A, Douglas R, Salt T, Akimoto M, Swaroop A, Sowden J and Ali R R 2006 Retinal repair by transplantation of photoreceptor precursors Nature 444 203–7
Mahadevappa M, Weiland J D, Yanai D, Fine I, Greenberg R J and Humayun M S 2005 Perceptual thresholds and electrode impedance in three retinal prosthesis subjects IEEE Trans. Neural Syst. Rehabil. Eng. 13 201–6
Maier S E, Hardy C J and Jolesz F A 1983 Brain and cerebrospinal fluid motion: real-time quantification with M-mode MR imaging Tohoku J. Exp. Med. 141 1–8
Marc R E and Jones B W 2003 Retinal remodeling in inherited photoreceptor degenerations Mol. Neurobiol. 28 139–47
Marc R E, Jones B W, Watt C B and Strettoi E 2003 Neural remodeling in retinal degeneration Prog.Retin.Eye.Res. 22 607–55
Marg E 1991 Magnetostimulation of vision: direct noninvasive stimulation of the retina and the visual brain Optom. Vis. Sci. 68 427–40
Marg E and Dierssen G 1965 Reported visual percepts from stimulation of the human brain with microelectrodes during therapeutic surgery Confin. Neurol. 26 57–75
Margalit E and Sadda S R 2003 Retinal and optic nerve diseases Artif. Organs 27 963–74
Margalit E et al 2002 Retinal prosthesis for the blind Surv. Ophthalmol. 47 335–56
Matsuzawa M, Potember R S and Krauthamer V 1994 Use of chemically patterned substrate to study directional effect of damaging electrical stimulation on cultured neuroblastoma cells Brain Res. 667 47–53
Merabet L B, Rizzo J F, Amedi A, Somers D C and Pascual-Leone A 2005 What blindness can tell us about seeing again: merging neuroplasticity and neuroprostheses Nat. Rev. Neurosci. 6 71–7
Milam A H, Li Z Y and Fariss R N 1998 Histopathology of the human retina in retinitis pigmentosa Prog.Retin.Eye Res. 17 175–205
Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P and Bamberg E 2003 Channelrhodopsin-2, a directly light-gated cation-selective membrane channel Proc. Natl Acad. Sci. USA 100 13940–5
Nakauchi K et al 2005 Transretinal electrical stimulation by an intrascleral multichannel electrode array in rabbit eyes Graefes. Arch. Clin. Exp. Ophthalmol. 243 169–74
Nashold B S Jr 1970 Phosphenes resulting from stimulation of the midbrain in man Arch. Ophthalmol. 84 433–5
Nehilla B J, Popat K C, Vu T Q, Chowdhury S, Standaert R F, Pepperberg D R and Desai T A 2004 Neurotransmitter analog tethered to a silicon platform for neuro-BioMEMS applications Biotechnol. Bioeng. 87 669–74
Neubauer A S, Priglinger S, Ullrich S, Bechmann M, Thiel M J, Ulbig M W and Kampik A 2001 Comparison of foveal thickness measured with the retinal thickness analyzer and optical coherence tomography Retina 21 596–601
Ohm J 1951 Der Nystagmus bei Blinden Albrecht Von Graefes Arch. Ophthalmol. 151 293–326
Olsen T W, Aaberg S Y, Geroski D H and Edelhauser H F 1998 Human sclera: thickness and surface area Am. J. Ophthalmol. 125 237–41
Pardue M T, Ball S L, Hetling J R, Chow V Y, Chow A Y and Peachey N S 2001 Visual evoked potentials to infrared stimulation in normal cats and rats Doc. Ophthalmol. 103 155–62
Pardue M T, Phillips M J, Yin H, Fernandes A, Cheng Y, Chow A Y and Ball S L 2005a Possible sources of neuroprotection following subretinal silicon chip implantation in RCS rats. Neural Eng. 2 S39–47
Pardue M T, Phillips M J, Yin H, Sippy B D, Webb-Wood S, ChowAYand BallSL 2005b Neuroprotective effect of subretinal implants in the RCS rat Invest. Ophthalmol. Vis. Sci. 46 674–82
Pelz J B and Canosa R 2001 Oculomotor behavior and perceptual strategies in complex tasks Vis. Res. 41 3587–96
Peterman M, Mehenti N, Bilbao K, Lee C, Leng T, Noolandi J, Bent S, Blumenkranz M and Fishman H 2003a The artificial synapse chip: a flexible retinal interface based on directed retinal cell growth and neurotransmitter stimulation Artif. Organs 27 975–85
Peterman M, Bloom D, Lee C, Bent S, Marmor M, Blumenkranz M and Fishman H 2003b Localized neurotransmitter release for use in a prototype retinal interface Invest. Ophthalmol. Vis. Sci. 44 3144–9
Peterman M C, Noolandi J, Blumenkranz M S and Fishman H A 2004 Localized chemical release from an artificial synapse chip Proc. Natl Acad. Sci. USA 101 9951–4
Peyman G, Chow A Y, Liang C, Chow V Y, Perlman J I and Peachey N S 1998 Subretinal semiconductor microphotodiode array Ophthalmic Surg. Lasers 29 234–41
Polyak S 1941 The Retina (Chicago, IL: The University of Chicago Press)
Potts A M and Inoue J 1969 The electrically evoked response (EER) of the visual system. II. Effect of adaptation and retinitis pigmentosa Invest. Ophthalmol. 8 605–12
Preuss T and Coleman G 2002 Human-specific organization of primary visual cortex: alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A Cereb. Cortex. 12 671–91
Purkinje J E 1823 Beobachtungen und Versuche zur Physiologie der Sinne. Ersted Bandehen, Prag Czech. J.G. Calve
Qiu X, Kumbalasiri T, Carlson S, Wong K, Krishna V, Provencio I and Berson D 2005 Induction of photosensitivity by heterologous expression of melanopsin Nature 433 745–9
Quinn R H and Miller S S 1992 Ion transport mechanisms in native human retinal pigment epithelium Invest. Ophthalmol. Vis. Sci. 33 3513–27
Rathnasingham R, Kipke D, Bledsoe S and McLaren J 2004 Characterization of implantable microfabricated fluid delivery devices IEEE Trans. Biomed. Eng. 51 138–45
Rattay F and Resatz S 2004 Effective electrode configuration for selective stimulation with inner eye prostheses IEEE Trans. Biomed. Eng. 51 1659–64
Rivolta C, Sharon D, DeAngelis M M and Dryja T P 2002 Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns Hum. Mol. Genet. 11 1219–27
Rizzo J 2006 Possible causes and biological impact of high stimulation thresholds/development of a wireless, first generation Boston Retinal Implant sub-retinal prosthesis The Eye and the Chip: Proc. World Congr. on Artificial Vision (Detroit, 15–17 June 2006)
Rizzo J F III, Wyatt J, Loewenstein J, Kelly S and Shire D 2003a Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials Invest. Ophthalmol. Vis. Sci. 44 5362–9
Rizzo J F III, Wyatt J, Loewenstein J, Kelly S and Shire D 2003b Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays Invest. Ophthalmol. Vis. Sci. 44 5355–61
Robblee L, Lefko J and Brummer S 1983 Activated iridium: An electrode suitable for reversible charge injection in saline solution J. Electrochem. Soc. 130 731–3
Rockland K S and Knutson T 2000 Feedback connections from area MT of the squirrel monkey to areas V1 and V2 J. Comp. Neurol. 425 345–68
Rockland K S, Saleem K S and Tanaka K 1994 Divergent feedback connections from areas V4 and TEO in the macaque Vis. Neurosci. 11 579–600
Ronner S F 1982 Prosthesis-related studies on visual cortex neurons Appl. Neurophysiol. 45 18–24
Rose T L and Robblee L S 1990 Electrical stimulation with Pt electrodes: VIII. Electrochemically safe charge injection limits with 0.2 ms pulses IEEE Trans. Biomed. Eng. 37 1118–20
Sachs H G, Schanze T, Brunner U, Sailer H and Wiesenack C 2005 Transscleral implantation and neurophysiological testing of subretinal polyimide film electrodes in the domestic pig in visual prosthesis development J. Neural Eng. 2 S57–64
Safadi M R, Washko F, Lagman A, Jaboro C, Auner G W, Iezzi R, McAllister J and Abrams G 2003 Development of a microfluidic drug delivery neural stimulating device for vision Invest. Ophthalmol. Vis. Sci. 44 (Suppl.) 5082 (abstract)
Sakaguchi H et al 2004 Transretinal electrical stimulation with a suprachoroidal multichannel electrode in rabbit eyes Japan. J. Ophthalmol. 8 256–61
Sakai T, Yoshitoshi T, Kawagoe M, Mizobuchi T and Kitahara K 1999 Expression of brain-derived neurotrophic factor gene in retina following vitreous tap Nippon Ganka Gakkai Zasshi. 103 271–6
Salat D H, Buckner R L, Snyder A Z, Greve D N, Desikan R S, Busa E, Morris J C, Dale A M and Fischl B 2004 Thinning of the cerebral cortex in aging Cereb. Cortex. 14 721–30
Santos A, Humayun M S, de Juan E Jr, Greenburg R J, Marsh M J, Klock I B and Milam A H 1997 Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis Arch. Ophthalmol. 115 511–5
Sauer B 1983 Quantitative analysis of the laminae of the striate area in man. An application of automatic image analysis. Hirnforsch. 24 89–97
Schanze T, Sachs H, Wiesenack C, Brunner U and Sailer H 2006 Implantation and testing of subretinal film electrodes in domestic pigs Exp. Eye Res. 82 332–40
Schiller P H 1977 The effect of superior colliculus ablation on saccades elicted by cortical stimulation Brain Res. 122 154–6
Schiller P and Tehovnik E 2002 Look and see: how the brain moves your eyes about Prog. Brain Res. 134 127–42
Shakhnovich A R, Ogleznev K Ya, Abakumova L Ya, Tishchenko L S and Razumovskii A E 1982 Phosphene formation during electrical stimulation of the visual cortex Hum. Physiol. 8 34–9
Schmidt M, Bak M J, Hambrecht F T, Kufta C V, O’Rourke D K O and Vallabhanath P 1996 Feasibility of a visual prothesis for the blind based on intracortical microstimulation of the visual cortex Brain 119 507–22
Schmidt S, Horch K and Normann R 1993 Biocompatibility of silicon-based electrode arrays implanted in feline cortical tissue J. Biomed. Mater. Res. 27 1393–9
Schneider K A, Richter M C and Kastner S 2004 Retinotopic organization and functional subdivisions of the human lateral geniculate nucleus: a high-resolution functional magnetic resonance imaging study J. Neurosci. 24 8975–85
Serruya M, Hatsopoulos N, Paninski L, Fellows M and Donoghue J 2002 Instant neural control of a movement signal Nature 416 141–2
Sjostrand J, Popovic Z, Conradi N and Marshall J 1999 Morphometric study of the displacement of retinal ganglion cells subserving cones within the human fovea Graefes Arch. Clin. Exp. Ophthalmol. 237 1014–23
Sobel I 1970 Camera models and machine perception AIM-21, Stanford Artificial Intelligence Laboratory (Palo Alto, California)
Stensaas S S, Eddington D K and Dobelle W H 1974 The topography and variability of the primary visual cortex in man. Neurosurg. 40 747–55
Stockman A and Sharpe L 2000 The spectral sensitivities of the middle-and long-wavelength-sensitive cones derived from measurements in observers of known genotype Vis. Res. 40 1711–37
Stone J L, Barlow W E, Humayun M S, de Juan E Jr and Milam A H 1992 Morphometric analysis of macular photoreceptor and ganglion cells in retinas with retinitis pigmentosa Arch. Ophthalmol. 110 1634–9
Strettoi E and Pignatelli V 2000 Modifications of retinal neurons in a mouse model of retinitis pigmentosa Proc. Natl Acad. Sci. USA 97 11020–5
Suzuki S, Humayun M S, Weiland J D, Chen S J, Margalit E, Piyathaisere D V and de Juan E Jr 2004 Comparison of electrical stimulation thresholds in normal and retinal degenerated mouse retina Japan. J. Ophthalmol. 48 345–9
Talalla A, Bullara L and Pudenz R 1974 Electrical stimulation of the human visual cortex; preliminary report Can. J. Neurol. Sci. 1 236–8
Tassiker G E 1956 US Patent 2760 483
Tehovnik E J, Slocum W M, Carvey C E and Schiller P H 2005 Phosphene induction and the generation of saccadic eye movements by striate cortex J. Neurophysiol. 93 1–19
Tehovnik E J, Slocum W M and Schiller P H 2003 Saccadic eye movements evoked by microstimulation of striate cortex Eur. J. Neurosci. 17 870–8
Tehovnik E J 1996 Electrical stimulation of neural tissue to evoke behavioral responses J. Neurosci. Methods. 65 1–17
Tomita T, Murakami M and Hashimoto Y 1960 On the R membrane in the frog’s eye: its localization, and relation to the retinal action potential J. Gen. Physiol. 43 81–94
Troyk P, Bak M, Berg J, Bradley D, Cogan S, Erickson R, Kufta C, McCreery D, Schmidt E and Towle V 2003 A model for intracortical visual prosthesis research Artif. Organs 27 1005–15
Tsuboi S, Pederson J and Toris C 1987 Functional recovery of retinal pigment epithelial damage in experimental retinal detachment Invest. Ophthalmol. Vis. Sci. 28 1788–94
Varma R, Bazzaz S and Lai M 2003 Optical tomography-measured retinal nerve fiber layer thickness in normal latinos Invest. Ophthalmol. Vis. Sci. 44 3369–73
Varma R, Skaf M and Barron E 1996 Retinal nerve fiber layer thickness in normal human eyes Ophthalmology 103 2114–9
Veraart C, Raftopoulos C, Mortimer J T, Delbeke J, Pins D, Michaux G, Vanlierde A, Parrini S and Wanet-Defalque M C 1998 Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode Brain Res. 813 181–6
Volker M, Shinoda K, Sachs H, Gmeiner H, Schwarz T, Kohler K, Inhoffen W, Bartz-Schmidt K U, Zrenner E and Gekeler F 2004 In vivo assessment of subretinally implanted microphotodiode arrays in cats by optical coherence tomography and fluorescein angiography Graefes Arch. Clin. Exp. Ophthalmol. 242 792–9
Vrabec F 1966 The temporal raphe of the human retina Am. J. Ophthalmol. 62 926–38
Vu T Q, Chowdhury S, Muni N J, Qian H, Standaert R F and Pepperberg D R 2005 Activation of membrane receptors by a neurotransmitter conjugate designed for surface attachment Biomaterials 14 1895–903
Wang L, Dong J, Cull G, Fortune B and Cioffi G A 2003 Varicosities of intraretinal ganglion cell axons in human and nonhuman primates Invest. Ophthalmol. Vis. Sci. 44 2–9
Wang D Y, Chan W M, Tam PO, Baum L, Lam D, Chong K K, Fan B J and Pang C P 2005 Gene mutations in retinitis pigmentosa and their clinical implications Clin. Chim. Acta 351 5–16
Warren D J, Fernandez E and Normann R A 2001 High-resolution two-dimensional spatial mapping of cat striate cortex using a 100-microelectrode array Neuroscience 105 19–31
Warren D J and Normann R A 2005 Functional reorganization of primary visual cortex induced by electrical stimulation in the cat Vis. Res. 45 551–65
Weiland J D, Liu W and Humayun M S 2005 Retinal prosthesis Ann. Rev. Biomed. Eng. 7 361–401
Wickelgren I 2006 A vision for the blind Science 312 1124–7
Wilke R, Kuttenkeuler C, Wilhelm B, Sailer H, Sachs H, Gabel V-P, Besch D, Bartz-Schmidt K and Zrenner E 2006 Subretinal chronic multi-electrode arrays in blind patients: perception of dots and patterns Invest. Ophthalmol. Vis. Sci. supplement 3202/B570
Wise K D, Anderson D J, Hetke J F, Kipke D R and Najafi K 2004 Wireless implantable microsystems: high-density electronic interfaces to the nervous system Proc. IEEE 9 76–97
Yagi T, Ito Y, Kanda H, Tanaka S, Watanabe M and Uchikawa Y 1999 Hybrid retinal implant: fusion of engineering and neuroscience Proc. 1999 IEEE Int. Conf. on Systems, Man and Cybernetics IV 382–5
Yagi T, Watanabe M, Ohnishi Y, Okuma S and Mukai T 2005 Biohybrid retinal implant Research and development update in 2005 IEEE Proc. 2005 2nd Int. Conf. Neural. Eng. 248–51
Yamauchi Y, Franco L M, Jackson D J, Naber J F, Ziv R O, Rizzo J F, Kaplan H J and Enzmann V 2005 Comparison of electrically evoked cortical potential thresholds generated with subretinal or suprachoroidal placement of a microelectrode array in the rabbit J. Neural Eng. 2 S48–S56
Zrenner E 2002a The subretinal implant: can microphotodiode arrays replace degenerated retinal photoreceptors to restore vision? Ophthalmologica 216 (Suppl. 1) 8–20
Zrenner E 2002b Will retinal implants restore vision? Science 295 1022–5
Zrenner E, Stett A, Weiss S, Aramant R B, Guenther E, Kohler K, Miliczek K, Seiler M and Haemmerle H 1999 Can subretinal microphotodiodes successfully replace degenerated photoreceptors? Vis. Res. 39 2555–67